Additional Reactions of importance
Diels-Alder Reaction
The Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene (referred to as the 'dienophile') to form a cyclohexene ring in a concerted mechanism by a pericyclic reaction. The diene must be in a s-cis conformation, s-trans conformations do not exhibit the reaction. This reaction is a thermally allowed, [4 + 2] cycloaddition, under Woodward-Hoffman rules [π4s+π2s]
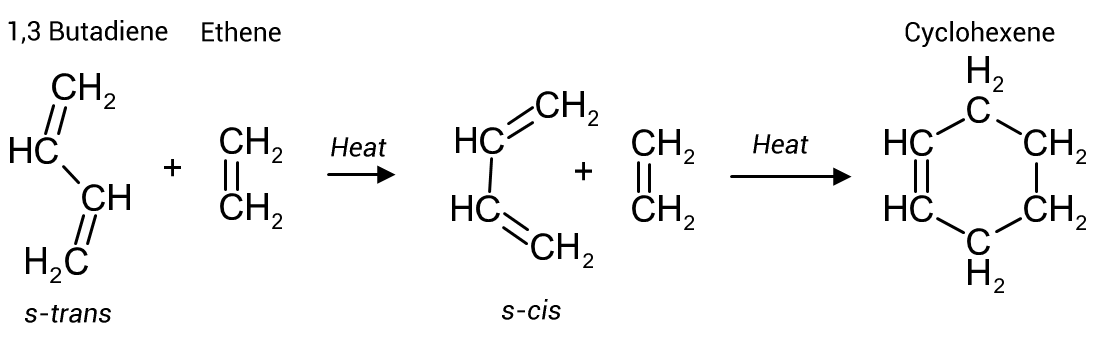
Figure 1, provides a simple example of the cycloaddition of ethene (as dienophile) to 1,3 butadiene.
At room temperature the configuration of 1,3 butadiene is s-trans whereas the Diels-Alder reaction requires the diene to be s-cis. This is accomplished by heating the butadiene which will rotate around the C2/C3 bond increasing the ratio of the s-cis configuration with which the ethene will readily react to provide the low energy 6 membered cyclohexene ring.
The rotation around the central C2/C3 sigma bond of the butadiene to the s-cis structure is unhindered by the carbon's small hydrogen atoms.
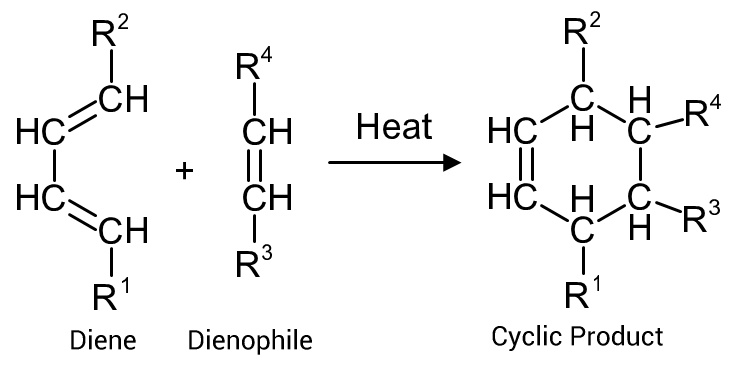
The reaction rate is increased, or retarded depending upon the substituent groups attached to either the diene of the dienophile.
Consider the the reaction in Figure 2
For substituent groups R¹/R² on the diene:
- Electron donating groups (EDG) enhance the reaction rate (e.g. alkyl groups, ethers, amines)
- Electron withdrawing groups (EWG) reduce the reaction rate (e.g. carboxyl, aldehydes, ketones)
For substituent groups R3/R4 on the dienophile:
- Electron withdrawing groups (EWG) enhance the reaction rate (e.g. carboxyl, aldehydes, ketones, phenyl)
- Electron donating groups (EDG) reduce the reaction rate (e.g. alkyl groups, ethers, amines)
Where there is an EWG on the diene and a EDG on the dienophile, the reaction is unlikely to proceed.
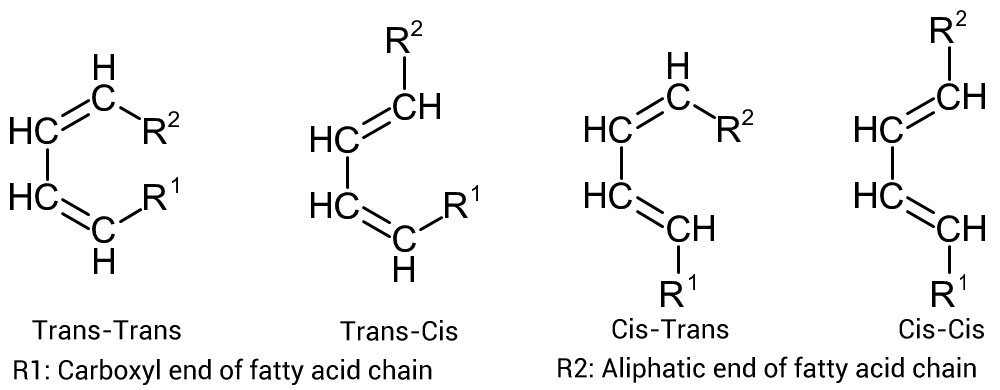
However circumstances change when the diene has larger substituent groups attached. When one is dealing with Diels-Alder reactions using conjugated fatty acids as the diene, matters change appreciably.
Figure 2, opposite shows the possible configurations for conjugated fatty acids based on the original conjugated cis/trans structure of the parent fatty acid molecule when converted to the required s-cis structure for Diels-Alder reactions.
When one is dealing with the 'normal' fatty acids, with a chain length of circa C18, Both R¹ and R² are bound to be quite bulky and steric hindrance of the approaching diene is likely to be substantial and hence the reaction temperature that the reaction will require will be quite high, especially when the conjugated double bonds are trans:trans.
Note that when dealing with the substituent groups on the diene and dienophile, the arrangement of the substituents will be stereospecific.
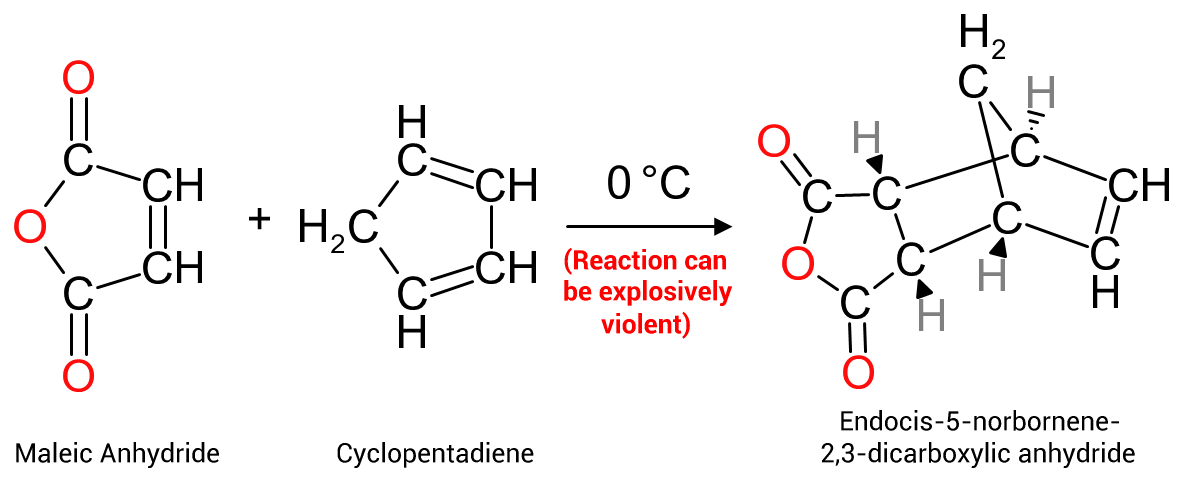
Of importance mainly to manufacturers of unsaturated polyesters, maleic anhydride may be reacted with cyclopentadiene to form endo cis-5-norbornene-2,3-dicarboxylic anhydride as per Figure 4 on the right.The bulky substituent group formed on the maleic portion of a polymer backbone aids in increasing the rigidity of the polymer, and also the HDT (Heat Distortion Temperature) of the cured polymer matrix. The modified properties make these resins makes them eminently suitable for use in the formulation of bulk, dough and sheet moulding compounds (BMC,DMC and SMC respectively)
Alder-ene reactions
The ene reaction, discovered by Kurt Alder, is a reaction between an alkene with an allylic hydrogen (a multiple carbon bond with a hydrogen atom in a position alpha to the bond, the 'ene') and and a compound with a double or triple carbon bond (the 'enophile').
These reactions were relatively unknown prior to 1943 but do present a method for implementing a new carbon-carbon σ bond between molecules.
This group reaction is also known as a Pericyclic reaction and requires high temperatures (± 200 °C) unless catalysed, for example with Lewis Acids.
Typical compounds used as enes are those unsaturated hydrocarbons that contain, olefinic, acetylenic, allenic, aromatic or carbon-heteroatom bonds (e.g. O₂, N₂, S, P).
An allylic hydrogen atom may be represented by figure 5, but-1-ene, where the 2 allylic hydrogens, those alpha to the double bond, are coloured red. These hydrogen atoms are easily transferable from one atom to another by virtue of the proximity of the double bond:

Meanwhile, the enophiles, contain π bonds with electron withdrawing substituents, not necessarily containing carbon-carbon π bonds but also be carbon-heteroatoms such as C=O, C=N, C≡P, or even hetero-hetero bonds such as N=N, O=O, N=O or O=S.
There are two important uses of this reaction mechanism in the manufacture of resins:
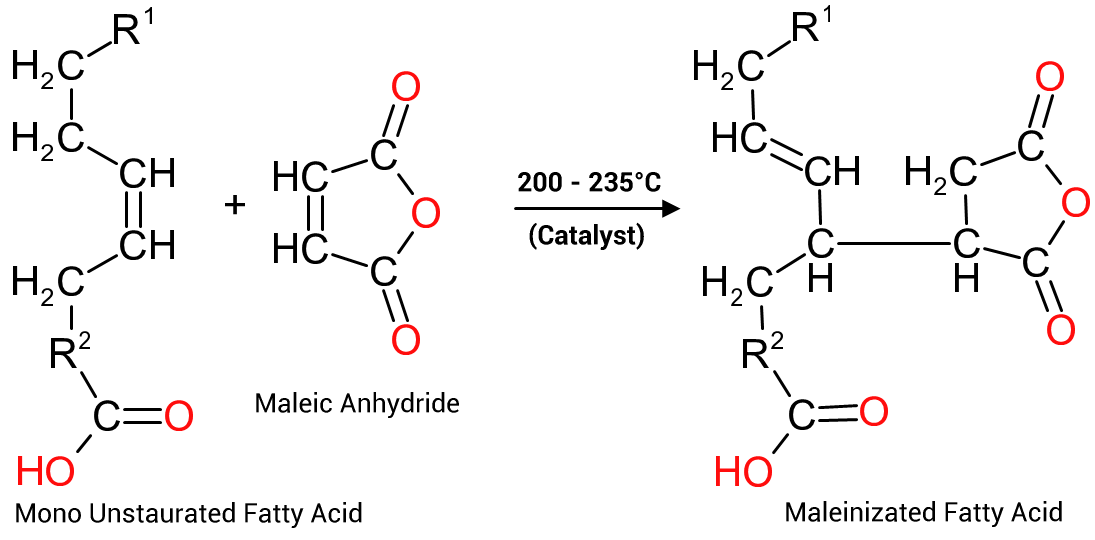
Maleinized Oils and Fatty Acids
The first reaction, the maleinization of oils and fatty acids is often used to increase the acid functionality of an unsaturated fatty acid (or oil or similar compound, like rosin for instance) from one to three, to increase the viscosity of the resin or, alternatively to add additional acid functionality to the resin to enhance water solubility or emulsifiability, as depicted in Figure 6 to the right.
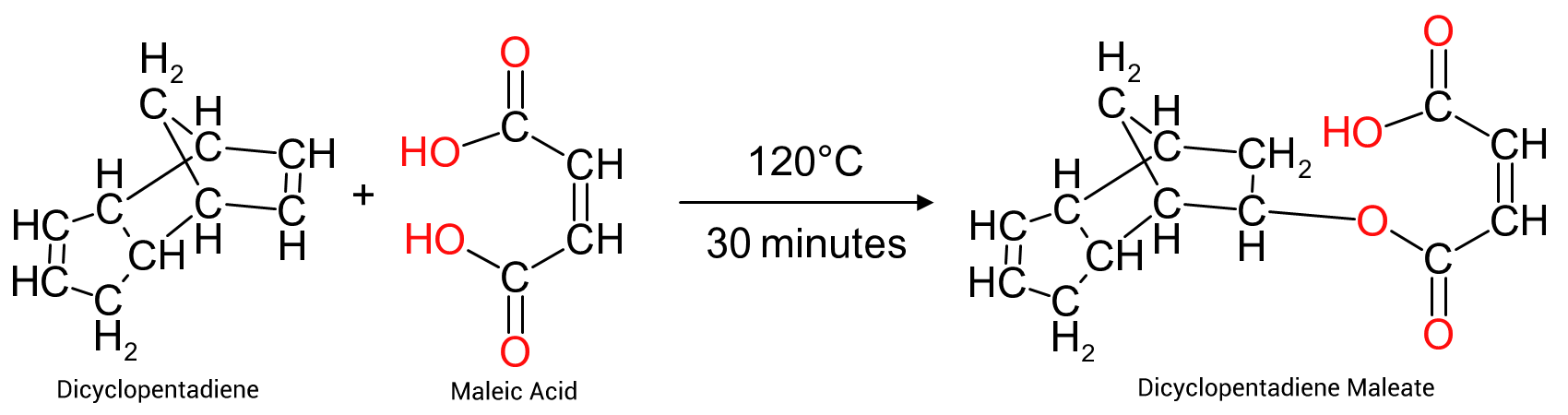
Dicyclopentadiene Maleate
The second reaction involves the formation of dicyclopentadiene maleate from maleic acid and DCPD (Figure 7). A reaction often used in the preparation of DCPD resins used in the formulation of DCPD resins designed for use as unsaturated polyesters. Maleic acid is formed in-situ by adding slightly more than an equimolar amount of water to maleic anhydride during the initial reaction stage at the start of the process.
Oil Modification
There are a number of ways to modify drying oils. These modifications to the raw oil precede that of the commerical production of alkyd resins (1926), and were developed to improve on the characteristics of the oil for use in paints and coatings. Increasing the viscosity of the oil improved both the viscosity of the oil and, in some cases, the drying performance
- Bodied Oils - a method of increasing the viscosity of the oil by means of using high temperatures (circa 280°C) to induce polymerisation of the oil molecules, though Diels Alder reactions, under an inert atmosphere, such as nitrogen or carbon dioxide, as outlined above.
- Blown Oils - A similar manufacturing technique, as per the bodied oils, except that oxygen (air) is injected into the hot oil to induce oxidative coupling between the oil molecules. Although the viscosity can be rapidly increased by this method, it results in the reduction of double bonds available for subsequent oxidative drying as a paint film.
- Boiled Oils - Pots of oil, open to the atmosphere, are heated, in combination with driers, to produce similar products to Bodied Oils, except that the process requires lower temperatures and time to produce the required viscosity of the polymer.
Modified oils of these varieties are still used today, mainly in the formulation of artist's paints, due to their combination of good pigment wetting properties, colour, gloss, flow, water resistance, and lack of penetration into porous substrates (such as artist's canvas).
The reason for mentioning these oil modifications in this series of reactions is that these processes can also occur in the manufacture of alkyd resins that are subjected to prolonged heating at high temperatures (heat bodying) or with the ingress of oxygen into the reactor (Blowing and/or Boiling due to the presence of catalysts, for example alcoholysis catalysts). If not done intentionally, such reactions can adversely affect the properties of the manufactured alkyd, most noticeably the cutting characteristics of the alkyd - a paint manufacturer's nightmare.